ASSALAMUALAIKUM..AND..HELLO TO ALL READERS
HANDSOME MR SHAMAN
BEAUTIFUL MRS WAN NADIAH
Before that, let us introduced our group members
LASTLY......
 |
| FARAH |
INTRODUCTION
A bioreactor is a vessel in which is carried out
a chemical process which involves organisms or biochemically active substances
derived from such organisms
Bioreactors
are commonly cylindrical, ranging in size from some liter to cube meters,and
are often made of stainless steel.
Bioreactor
design is quite a complex engineering task. Under optimum conditions the
microorganisms or cells will reproduce at an astounding rate. The vessel's
environmental conditions like gas (i.e., air, oxygen, nitrogen, carbon dioxide)
flowrates, temperature, pH and dissolved oxygen levels, and agitation speed
need to be closely monitored and controlled.
METHODOLOGY
Preparation of media and reagents
1.Yeast extract,
peptone and glucose is weighed in the ratio of 3:1:1 and is added into a flask.
2.300ml of
distilled water is added.
3.The solution is
stirred until homogeneous solution is obtained.
Figure 1
5.The media is autoclaved at 121oC
with 15 minutes retention time.
6.The flask is
stored in an incubator shaker under the conditions of 200rpm and 30oC
overnight.
Setting up
bioreactor
1.The bioreactor is cleaned with distilled water to remove any residue
from previous usage.
2.Upon cleaning, the orifice of the sparger is inspected for any
blockage by running water through the air sparger tubes. (figure 2)
Figure 2
Figure 2
3.The agitator and agitator driver is lubricated using glysol solution until the lubricant flooded at the top of the drive.
Figure 3
4.The bioreactor is assembled. Glass vessel is connected to the base unit, lifting with the handles of the support frame and the stud is placed at the back of the vessel into the metal fork on the support frame.
5.The drive arm is
lowered into the horizontal position.
6.The side shield
is replaced to keep it out together with the base unit.
7.The exit gas
cooler is fit into a free port and the water connecting tubing is checked for
length and connections are made ready for the next day.
8.The pO2
electrode is located and the green plastic end cap is removed. The bottom metal
section is unscrewed and the membrane cartridge inside is checked whether has
liquid electrolyte in it. If not, up to half is topped from the bottle
provided.
9. The pO2
electrode is fit into the vessel loosely. The top plastic cap is removed from
the electrode.
10. the connection
point between the vessel and head plate is smeared with high vaccum grease
(figure 4).
Figure 4
11. All internal part of the bioreactor(vessel, sparger, impeller, agitator and head plate) is sprayed with alcohol solution (figure 5). The head plate and vessel is then connected.
Figure 5
12. Two reagent
bottles are prepared (anti-foam and base). Anti-foam is poured into one of the
reagent bottles while the one for the base is filled with distilled water prior
to autoclave.
13. Yeast extract:
Peptone: Glucose (YEPG) medium is prepared with the same ratio of 3:1:1 and
distilled water is added until the volume reaches 900ml. The medium is then
added into the vessel.
14. All openings,
filters and pump are wrapped with aluminium foil and all tubings are clamped
before autoclave. (Figure 6)
Figure 6
Inoculation into the media and fermentation.
1. After
autoclaving, all aluminium foil is unwrapped and all clamps are removed.
2. Base is filled
into the reagent bottles and both the pumps of the reagent bottles are fitted
to the correct motors and the tubing connected to the multi-way inlet and
clamped closely. (figure 8)
Figure 8
3. Temperature, pH and pO2 electrode are fitted into the vessel and are calibrated.
4. Inoculum is
poured into the vessel under aseptic condition.
5.Speed is
adjusted to 300rpm and cascade, pH and pO2 are all on. Fermentation
is run and the sample is collected every 2 hours. (figure 9)
RESULT
Times
|
OD
|
Blank
|
0.000
|
3.30pm
|
0.148
|
5.30pm
|
0.793
|
7.30pm
|
0.803
|
9.30pm
|
0.824
|
11.30pm
|
0.811
|
1.30am
|
0.940
|
3.30am
|
0.934
|
5.30am
|
0.901
|
7.30am
|
0.324
|
9.30am
|
0.171
|
RESULT OF GLUCOSE READING
TIME
|
CONDITION
|
READING (mg/dl)
|
3.30pm
|
267
|
|
5.30pm
|
246
|
|
7.30pm
|
226
|
|
9.30pm
|
Diluted
|
182
|
11.30pm
|
Diluted
|
Low
|
1.30am
|
Diluted
|
Low
|
3.30am
|
Diluted
|
low
|
5.30am
|
Diluted
|
low
|
7.30am
|
Diluted
|
Low
|
9.30am
|
Diluted
|
low
|
DISCUSSION
The
inoculum that used in the experiment was 150ml of YEPG, 1% yeast extract
(10mg/ml), 2% peptone (20mg/ml) and 2% glucose (20mg/ml). While the culture
medium used was 10 times of the materials that used to prepare the inoculum.
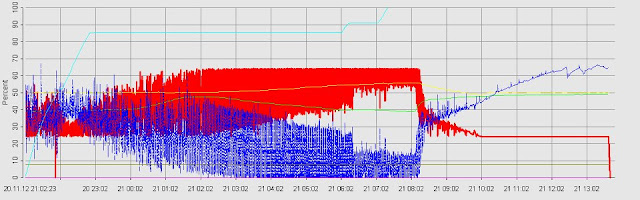
According to the results of my group
obtained, the OD of the yeast (Saccharomyces cerevisiae) was showing increased
from 0 hour (OD= 0.148) to 10 hours (OD= 0.940), this indicated the log phase
of the yeast cells. After 10 hours growing, the yeast cells were reached the
stationary phase from 10 hours (OD= 0.940) until 14 hours (OD= 0.901). After
the stationary phase ended, the yeast cells were reached the death phase in
which the OD was sharply decrease from 0.901 to 0.321 and finally reached
0.171. The growth curve of any organisms including the yeast cells that we used
in this experiment- Saccharomyces cerevisiae are following the sigmoid curve,
which start from lag phase, log phase, stationary phase and finally reach the
death phase.
From
the results we also can see that the glucose concentration in the culture
medium was gradually decreased from 267mg/dl to low concentration
(undetectable). This is because the yeast cells required the glucose as the
substrate to carry out aerobic fermentation to increase the biomass. Depleted
in the glucose will decrease or stop the growth of yeast.
Alkali was used in the experiment because
need to regulate and maintain the pH of the medium at pH 6, which is slightly
acidic condition. Due to the production of acetic acid during the aerobic
fermentation by the yeast, the pH of the medium will dropped to below 6, the
unsuitable and too acidic pH will affected the yeast growth, thus the alkali
was playing the role in increase the pH of the medium back to pH 6, an optimum
pH for the optimum growth of yeast.
Anti foam agent was also been used in the
experiment, this is because a lot of foam will be produced during the
fermentation process, especially in the aerobic fermentation like we carried
out in this experiment. The role of this anti foam agent is to reduce the foam
formation in the culture medium. The foam will affect the yeast cells growth
and make the medium over flow from the vessel, results in the contamination and
waste of the product.
Because
the process of fermentation we carried out was aerobic, which mean that oxygen
was required to increase the yeast biomass, so the inlet air was needed to pump
into vessel to succeed the fermentation process. 3.3vvm was the level that we
used in the experiment in order to pump in the sufficient amount of oxygen into
the medium for yeast consumption. The inlet and outlet air were required filter
(the pore sizes were 0.2 nanometer) to filter out the microbes normally made up
of bacteria and fungus from entering into the vessel which will cause
contamination and escaped out to the surrounding air. Exit air cooler was used
to condense back the medium which had been evaporated back into the vessel,
this is to prevent the excess products loss, even though the efficiency of the
cooler is around 95%. Insufficient in oxygen will lead the yeast to carry our
anaerobic fermentation, which convert the glucose into bioethanol, no biomass
will produce.
The speed of agitation applied during the
shake flask culture will influence the maximum growth rate of the yeast when
cultured in the bioreactor. The faster the speed of agitation during the shake
flask culture (300rpm), the faster the yeast increase in the biomass. If the
rpm was at 200 during the shake flask culture, the yeast will show slow growing
when cultured in the bioreactor which the speed of the impellers also
maintained at 200rpm. This is due to the insufficient obtained in the oxygen
and also glucose by the yeast to achieve optimum growth.
The optimum temperature for the yeast to
grow is 30 degree celcius. The heat will be produced during the aerobic
fermentation, which the temperature of the medium will increase. Unsuitable
temperature will affect the yeast growth, so the jacket water inlet is required
to regulate and maintain the medium’s temperature at 30 degree celcius.
CONCLUSION
The setting up of bioreactor needed
to be done carefully to ensure smooth and successful fermentation process.
Aseptic technique is vital to ensure maximum yield of product by preventing
contamination. At the end of this experiment, better understanding on
bioreactor operation was obtained and experiences were gained for the handling
of bioreactor during fermentation process.
REFERENCE:
1.
1.Transgalactic Ltd, 2005. What Is Bioreactor?. [online] Available
at: <http://www.bionewsonline.com/o/what_is_bioreactor.htm]. [Accessed 5
November 2012 ]
THANK YOU........




















Nice writing, Thank you, guys :)
ReplyDelete